Stomach cancer starts in the lining of your stomach and impacts over 26,500 people in the U.S. every year. Hearing the words ‘you have stomach cancer’ can be scary, but learning about what comes next—from spotting early signs to life after treatment—can help you and your loved ones feel more in control. In this guide, we’ll break down the basics: how doctors test for it, today’s treatments, and simple ways to stay healthy during recovery.
Understanding Stomach Cancer Diagnosis
Stomach cancer’s insidious nature lies in its ability to masquerade as benign digestive complaints. Over 60% of cases are diagnosed at Stage III or IV, largely due to non-specific early symptoms such as intermittent dyspepsia (indigestion) or epigastric discomfort—issues often dismissed as stress or dietary indiscretion. This diagnostic lag reduces 5-year survival rates from 70% in localized cases to a stark 6% in metastatic disease.
The Diagnostic Triad: Tools Uncovering Hidden Disease
1. Upper Endoscopy (Esophagogastroduodenoscopy)
- Procedure: A flexible endoscope with a high-resolution camera is inserted transorally, allowing real-time visualization of the gastric mucosa. Advanced techniques like narrow-band imaging (NBI) enhance detection of subtle lesions by highlighting vascular patterns.
- Utility: Identifies erythema (redness), ulcerations, or mass lesions. A 2020 Gastrointestinal Endoscopy study found endoscopy detects 92% of proximal gastric tumors but only 68% of diffuse-type cancers in early stages.
- Limitations: Operator-dependent; flat lesions (e.g., signet-ring cell carcinomas) may evade detection.
2. Biopsy & Histopathological Analysis
- Protocol: Performed during endoscopy, multiple samples (6-8) are taken from suspicious areas. The Sydney System guides biopsy sites for optimal yield.
- Lab Workflow:
- HER2 Testing: Immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH) identifies HER2 overexpression (seen in 10-15% of cases), guiding trastuzumab therapy.
- Molecular Profiling: Next-gen sequencing (NGS) assesses biomarkers like PD-L1 for immunotherapy eligibility.
- Pitfalls: False negatives occur in diffusely infiltrating tumors without distinct mass lesions.
3. Multimodal Imaging: Mapping Disease Extent
- CT Scan: The cornerstone for staging, offering 85-90% accuracy in detecting lymph node involvement. Dual-phase contrast protocols differentiate T1-T2 tumors.
- PET/CT: Superior for detecting distant metastases (e.g., hepatic, peritoneal) but limited in signet-ring cell carcinoma due to low FDG avidity.
- Endoscopic Ultrasound (EUS): Critical for assessing tumor depth (T-stage) and perigastric lymph nodes.
The Human Cost of Delayed Detection
A 2022 Journal of Clinical Oncology analysis revealed that 43% of patients initially dismissed symptoms for 3+ months, attributing them to “acid reflux” or “aging.” Case in point:
- Patient A: A 58-year-old male with 6 months of “burning” epigastric pain treated with OTC antacids. Diagnosis: Stage IV adenocarcinoma with peritoneal carcinomatosis.
- Patient B: A 47-year-old female who sought care after 3 weeks of hematemesis (vomiting blood). Diagnosis: Stage IIA intestinal-type adenocarcinoma, curative resection achieved.

Why Early Detection Demands a Paradigm Shift
- Survival Disparities: Early-stage (T1a) tumors treated endoscopically (ESD/EMR) boast 5-year survival >90%, versus <30% for Stage IIIB.
- Economic Burden: Late-stage care costs 3.8x more than early treatment (per NIH data), driven by prolonged chemo/immunotherapy.
- Psychosocial Impact: Advanced disease correlates with higher rates of treatment-related anxiety (62% vs. 28% in early-stage patients).
Breaking Barriers: Strategies for Timely Diagnosis
- Risk-Stratified Screening: Advocate for endoscopic surveillance in high-risk cohorts (e.g., H. pylori-positive patients, familial GC families).
- Symptom Algorithms: Tools like the ABC score (Age, Blood tests, Clinical signs) stratify dyspepsia patients for urgent endoscopy.
- Public Awareness: Campaigns reframing “red flag” symptoms (e.g., *unintentional 5% weight loss in 6 months = immediate workup*).
Why It Matters: Early detection improves survival rates, but late-stage diagnoses are common. If you experience persistent symptoms like unexplained weight loss or blood in stool, seek medical evaluation immediately.
Stomach Cancer Treatment Options
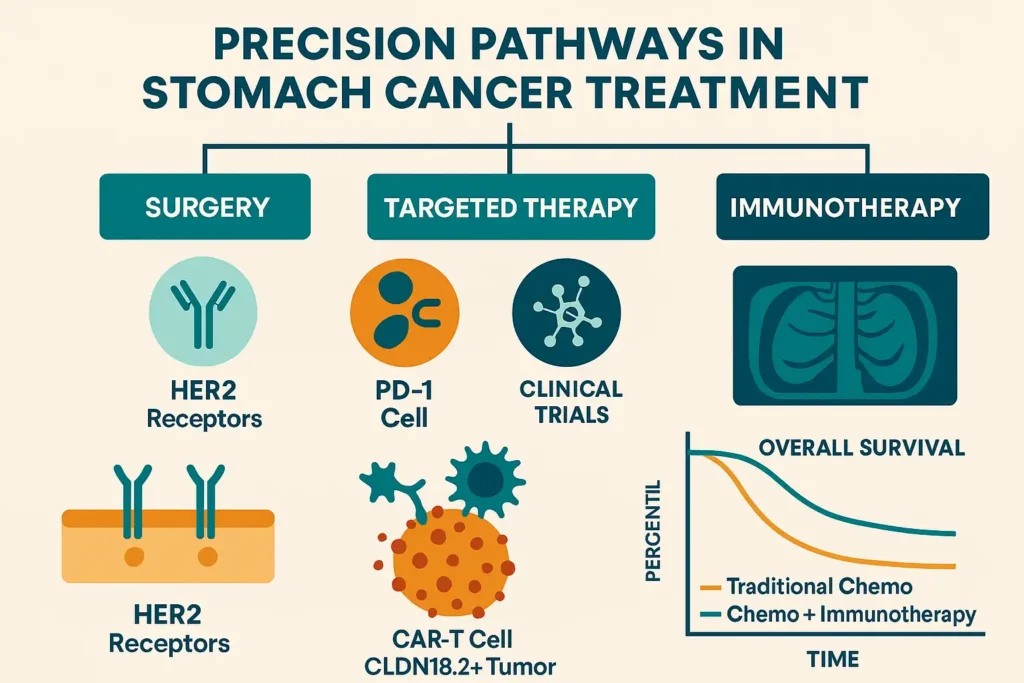
Treatment strategies for gastric adenocarcinoma and other stomach malignancies are dictated by tumor stage, molecular profile, and patient fitness. The paradigm has shifted from one-size-fits-all protocols to precision oncology, with therapies increasingly guided by biomarkers like HER2, PD-L1, and Claudin 18.2 (CLDN18.2). Below, we dissect current standards and emerging frontiers.
1. Surgical Intervention: Curative Intent and Palliation
Indications:
- Early-stage (T1a-T2N0): Endoscopic resection (ESD/EMR) or laparoscopic partial gastrectomy.
- Locally advanced (T3-T4/N+): Total/subtotal gastrectomy with D2 lymphadenectomy (removal of ≥15 nodes).
Procedural Nuances:
- Minimally Invasive (MIGS): Robotic-assisted gastrectomy reduces blood loss and hospital stays vs. open surgery (per 2023 Annals of Surgery meta-analysis).
- Palliative Surgery: For obstruction/bleeding in Stage IV, bypass procedures (e.g., gastrojejunostomy) improve quality of life.
Survival Data:
- R0 resection (no residual tumor) achieves 5-year survival of 50-60% in Stage IIB-IIIB.
- Post-op morbidity: 20-30% (anastomotic leaks, dumping syndrome).
2. Systemic Therapy: Chemotherapy and Targeted Agents
Neoadjuvant (Pre-Surgery):
- FLOT Protocol: 4 cycles of 5-FU, leucovorin, oxaliplatin, docetaxel. FLOT4-AIO trial showed 35% pathologic complete response (pCR) in resectable tumors.
- Immunotherapy Combo: KEYNOTE-585 trial testing pembrolizumab + chemo in locally advanced cases.
Adjuvant (Post-Surgery):
- CAPEOX: Capecitabine + oxaliplatin reduces recurrence by 20% in Stage II/III (CLASSIC trial).
Metastatic Disease:
- First-line:
- HER2+: Trastuzumab + FLOT (ToGA trial: 13.8 vs. 11.1 months survival).
- HER2-: FOLFOX/CAPEOX + nivolumab (CheckMate 649: 13.8 vs. 11.6 months).
- Second-line: Ramucirumab (VEGFR2 inhibitor) + paclitaxel (RAINBOW trial: 9.6 months OS).
3. Radiation Therapy: Precision Targeting
Role:
- Pre-op: 45-50 Gy radiation + chemo (CROSS regimen) shrinks tumors for R0 resection.
- Post-op: Reduces locoregional recurrence in node-positive cases (ARTIST II trial).
- Palliative: Eases pain/bleeding in unresectable tumors.
Innovations:
- IMRT/Proton Therapy: Minimizes toxicity to adjacent organs (e.g., heart, kidneys).
4. Immunotherapy: Unleashing the Immune System
Checkpoint Inhibitors:
- PD-1/PD-L1 Blockers: Pembrolizumab (KEYNOTE-062) approved for PD-L1 CPS ≥1 tumors.
- CTLA-4 Inhibitors: Limited efficacy but under study in combo regimens.
Biomarker-Driven Success:
- MSI-H/dMMR Tumors: Pembrolizumab achieves 45% objective response rate (ORR) vs. 15% with chemo.
- EBV+ Tumors: Emerging sensitivity to PD-1 inhibitors (20% ORR in phase II trials).
5. Emerging Frontiers: The Genomic Revolution
Liquid Biopsies: ctDNA analysis detects minimal residual disease (MRD) post-surgery, guiding adjuvant therapy.
CAR-T Cell Therapy: Early trials targeting CLDN18.2 (NCT04404595) show 50% ORR in refractory cases.
ADC Breakthroughs:
- Zolbetuximab (anti-CLDN18.2): GLOW trial improved PFS by 48% in CLDN18.2+ metastatic GC.
- Enhertu (DS-8201): HER2-low tumors saw 37% ORR in DESTINY-Gastric01.
Case Study: Personalized Therapy in Action
Patient Profile: 52F, Stage IV HER2+ diffuse-type gastric cancer with liver metastases.
- First-line: Trastuzumab + FLOT (partial response).
- Progression: Switch to Enhertu (HER2 ADC) – 8-month remission.
- ctDNA Monitoring: RAS mutation emergence prompted ramucirumab + irinotecan.
Emerging Trends: Clinical trials are exploring personalized treatments based on genetic mutations, offering hope for refractory cases.
Living with Stomach Cancer: Diet, Care, and Mental Health
Post-treatment life after stomach cancer demands a meticulous, multidisciplinary approach to restore physical health and emotional well-being. With 30-50% of survivors facing long-term nutritional, psychological, or functional challenges, proactive management is key to reclaiming quality of life. Below, we explore evidence-based strategies to navigate this new normal.
1. Nutritional Rehabilitation: Beyond “Small, Frequent Meals”
The Science of Dumping Syndrome
Common after gastrectomy, dumping syndrome occurs when undigested food rapidly enters the small intestine, triggering vasomotor symptoms (sweating, dizziness) and gastrointestinal distress (diarrhea, cramps).
- Early Dumping (30 mins post-meal): Caused by fluid shifts; managed via low-glycemic-index carbs (quinoa, oats) and avoiding liquids 30 mins before/after eating.
- Late Dumping (2-3 hrs post-meal): Hypoglycemia-driven; countered with protein-rich snacks (Greek yogurt, hard-boiled eggs).
Dietitian-Driven Protocols
- Phase 1 (Weeks 1-6): Liquid/pureed diets (e.g., blended soups, protein shakes) to prevent anastomotic stress.
- Phase 2 (Weeks 7-12): Soft solids (mashed avocado, steamed fish) with enzyme supplements (Creon) to aid fat absorption.
- Long-Term: High-protein (1.2-1.5g/kg/day), low-refined-sugar meals. Vitamin B12 injections (monthly) combat pernicious anemia.
Supplements & Monitoring
- Essential: Iron, calcium, zinc, and vitamin D (malabsorption risk).
- Avoid: Alcohol, carbonated drinks, and spicy foods (mucosal irritants).
- Tools: Apps like MyFitnessPal track macros; continuous glucose monitors (CGMs) manage reactive hypoglycemia.
2. Survivorship Care: Vigilance Against Recurrence
Surveillance Guidelines (NCCN 2023)
- Imaging: Contrast-enhanced CT abdomen/pelvis + chest every 3-6 months (Years 1-3), annually thereafter.
- Lab Work: CBC (anemia), LFTs (liver mets), and tumor markers (CEA, CA19-9)—though non-specific.
- Endoscopy: Annual EGD for retained stomach remnants; biopsy any mucosal irregularities.
Managing Late Effects
- Bile Reflux: Treated with ursodeoxycholic acid or surgical diversion (Roux-en-Y).
- Osteoporosis: DEXA scans biannually; bisphosphonates if T-score <-2.5.
- Fatigue: Rule out hypothyroidism (TSH testing); prescribe graded exercise therapy (GET).
The Role of Precision Monitoring
- ctDNA Testing: Detect molecular residual disease (MRD) 6 months post-op; linked to 12x higher relapse risk.
- Germline Testing: CDH1/Lynch syndrome carriers need annual breast/MRI screening.
3. Mental Health: Breaking the Silence
The Invisible Burden
- Prevalence: 42% of survivors report clinical anxiety/depression (per Journal of Cancer Survivorship).
- Triggers: Body image struggles (weight loss/scars), fear of recurrence (FOR), and financial toxicity.
Evidence-Based Interventions
- CBT (Cognitive Behavioral Therapy): Reduces FOR by reframing catastrophic thoughts (6-week programs show 50% symptom reduction).
- Pharmacotherapy: SSRIs (e.g., escitalopram) improve mood and chemotherapy-induced neuropathy.
- Mind-Body Practices: Yoga nidra (yogic sleep) lowers cortisol by 28%; acupuncture aids chemo-brain fog.
Community & Advocacy
- Peer Support: CancerCare’s Stomach Cancer Group offers virtual meetups; No Stomach For Cancer connects patients globally.
- Advocacy: Share stories via #StomachCancerAwareness; lobby for workplace accommodations (FMLA).
Case Study: A Survivor’s Journey
Maria, 58: Post-subtotal gastrectomy for Stage IIA adenocarcinoma.
- Nutrition: Uses digestive enzymes + 6 mini-meals daily; HbA1c stabilized at 5.8% with CGM.
- Survivorship: Year 2 surveillance detected peritoneal recurrence via ctDNA; now in remission on trastuzumab-deruxtecan.
- Mental Health: Joined a CBT group; practices mindfulness via Calm app.
Pro Tip: Keep a symptom journal to track side effects like fatigue or nausea and share updates with your oncology team.
Prevention and Risk Reduction
While stomach cancer’s complex etiology means absolute prevention isn’t guaranteed, 50-75% of cases are linked to modifiable lifestyle and environmental factors. By targeting known carcinogenic pathways—from microbial infections to dietary carcinogens—we can significantly mitigate risk. Below, we unpack actionable, evidence-based strategies rooted in global research (WHO, NCI) and clinical trials.
1. Eradicate H. pylori: The Prime Preventable Trigger
The Pathogen-Cancer Link
- Stats: H. pylori causes 65-80% of non-cardia gastric cancers via chronic inflammation → atrophic gastritis → dysplasia.
- Screening: Endorse stool antigen tests or urea breath tests for high-risk groups (familial GC, immigrants from high-prevalence regions like East Asia).
Treatment Protocols
- First-Line Therapy: Bismuth quadruple therapy (PPI + bismuth + metronidazole + tetracycline x14 days) achieves 90% eradication (Maastricht VI/2022).
- Post-Treatment Confirmation: Repeat testing 4 weeks post-therapy to confirm eradication.
Vaccine Horizon: Phase III trials for recombinant H. pylori vaccines (e.g., VacA/CagA antigens) show 68% efficacy in preventing colonization.
2. Dietary Defense: From Plate to Protection
Culinary Carcinogens to Avoid
- Nitrosamines: Found in processed meats (bacon, sausages) and pickled foods; convert to DNA-damaging compounds in acidic stomachs.
- High-Salt Diets: >5g/day accelerates H. pylori virulence and induces mucosal damage (per 2021 Gut meta-analysis).
Cancer-Fighting Foods
- Cruciferous Vegetables: Sulforaphane in broccoli sprouts inhibits H. pylori biofilm formation.
- Allium Family: Garlic (allicin) and onions (quercetin) reduce gastric cancer risk by 30% (EPIC study).
- Berries: Ellagic acid blocks COX-2, an enzyme overexpressed in gastric tumors.
Anti-Cancer Meal Plan
- Breakfast: Oatmeal + flaxseed + blueberries.
- Lunch: Grilled salmon + kale salad + turmeric-roasted sweet potatoes.
- Dinner: Miso soup (fermented soy) + stir-fried bok choy + quinoa.
3. Smoking Cessation: Breaking the Toxic Cycle
Mechanisms of Harm
- Tobacco smoke contains 70+ carcinogens (e.g., NNK, PAHs) that induce DNA adducts in gastric mucosa.
- Smoking synergizes with H. pylori, increasing corpus-predominant gastritis risk 4-fold.
Quitting Strategies
- Pharmacotherapy: Varenicline (Chantix) + nicotine patches yield 33% long-term abstinence (Cochrane Review).
- Behavioral Support: CDC’s 1-800-QUIT-NOW offers free counseling; apps like QuitGenius use CBT.
Impact: Quitting for 10+ years normalizes risk to near never-smoker levels.
4. Beyond the Basics: Overlooked Risk Modulators
Weight & Metabolic Health
- Visceral fat secretes IL-6 and leptin, driving chronic inflammation. A BMI >30 raises risk by 23% (NCI-SEER data).
- Fix: Aim for waist circumference <35” (women)/<40” (men); prioritize HIIT workouts to reduce visceral adiposity.
Alcohol Moderation
- Threshold: >3 drinks/day increases risk 1.8x; acetaldehyde (ethanol metabolite) is a Group 1 carcinogen.
- Safer Choices: Opt for resveratrol-rich red wine (<1 glass/day) or abstain.
Occupational Hazards
- Asbestos workers, coal miners, and metal industry workers face elevated risks. Advocate for PPE and regular EGD screening.
5. Comorbidity Management: The Surgery Survival Link
The 2018 Landmark Study
A JAMA Surgery analysis of 12,000 gastrectomy patients revealed:
- COPD Patients: 2.5x higher risk of anastomotic leaks.
- Diabetics: 3x higher surgical site infections.
Action Steps
- Prehab Programs: 4-6 weeks of aerobic conditioning (e.g., 150 mins/week brisk walking) + protein optimization (1.5g/kg/day) cut complications by 40%.
- Glycemic Control: A1C <7% pre-surgery via SGLT2 inhibitors or GLP-1 agonists.
Case Study: Risk Reduction in Action
Raj, 49: South Asian male with H. pylori+ chronic gastritis and BMI 34.
Outcome: Normalized gastric mucosa on repeat EGD; 18% weight loss. Now advocates for community H. pylori screening.
Intervention: Eradication therapy (quadruple regimen) + Mediterranean diet + 12-week smoking cessation program.
Did You Know? A 2018 study found patients with lung disease face higher post-surgery complications—highlighting the need for holistic health management.
FAQs
Q1: Is stomach cancer hereditary?
A1: Approximately 5–10% of stomach cancer cases are linked to inherited genetic mutations. Key syndromes include:
- Hereditary Diffuse Gastric Cancer (HDGC): Caused by CDH1 mutations, carrying a 70–80% lifetime risk of stomach cancer. Prophylactic gastrectomy is recommended for carriers.
- Lynch Syndrome: Mutations in *MLH1/MSH2* increase risk 8–10x, with annual surveillance endoscopy advised starting at age 30–35.
- Familial Adenomatous Polyposis (FAP) and Li-Fraumeni Syndrome: Less common but warrant genetic counseling.
Action Step: Families with ≥2 relatives diagnosed or early-onset cases (<50 years) should pursue germline testing (NCCN guidelines).
Q2: Can stomach cancer recur after treatment?
A2: Yes. Recurrence rates vary by stage:
- Stage I: 5–10% risk within 5 years.
- Stage III: 40–60% risk, often metastasizing to peritoneum or liver.
- Stage IV: Near-universal recurrence; palliative care focuses on slowing progression.
Surveillance Protocol: - Imaging: CT scans every 3–6 months for 3 years.
- Tumor Markers: CEA/CA19-9 tracked quarterly.
- ctDNA Testing: Detects molecular residual disease (MRD) 6–12 months post-surgery, predicting relapse 6–9 months earlier than imaging.
Q3: What are the earliest warning signs of stomach cancer?
A3: Early symptoms are subtle but include:
- Persistent indigestion unresponsive to antacids.
- Early satiety (feeling full after small meals).
- Mild upper abdominal pain.
Red Flags: Unexplained weight loss (>5% in 6 months) or iron-deficiency anemia warrants immediate endoscopy.
Q4: How does H. pylori infection increase stomach cancer risk?
A4: H. pylori causes chronic inflammation, leading to:
- Atrophic Gastritis: Loss of acid-producing cells (50% risk progression in 10 years).
- Intestinal Metaplasia: Pre-cancerous stomach lining changes (3–5% annual progression to cancer).
Prevention: Eradication therapy (bismuth quadruple regimen) reduces cancer risk by 35% (Maastricht VI Consensus).
Q5: What dietary changes lower stomach cancer risk?
A5: Prioritize:
- Antioxidant-Rich Foods: Berries, leafy greens (reduce oxidative DNA damage).
- Fermented Foods: Kimchi, miso (contain probiotics that inhibit H. pylori).
- Avoid: Processed meats (nitrites/nitrosamines) and >5g salt/day (doubles H. pylori virulence).
Study Insight: A Mediterranean diet lowers risk by 33% (EPIC cohort study).
Q6: Are new treatments improving survival rates?
A6: Breakthroughs include:
- Immunotherapy: Pembrolizumab boosts 3-year survival in PD-L1+ tumors to 22% vs. 8% with chemo (KEYNOTE-062).
- Antibody-Drug Conjugates (ADCs): Enhertu (DS-8201) achieves 40% response in HER2-low metastatic cases.
- CLDN18.2-Targeted CAR-T: Early trials show 50% tumor regression in refractory patients.
Q7: How does smoking impact stomach cancer risk?
A7: Smoking:
- Doubles risk for cardia (upper stomach) tumors.
- Synergizes with H. pylori, accelerating mucosal damage.
- Releases carcinogens (e.g., NNK) that mutate TP53 tumor suppressor genes.
Quitting Benefit: Risk normalizes to non-smoker levels after 10+ years cessation.
Q8: What mental health support exists for survivors?
A8: Resources include:
- Cognitive Behavioral Therapy (CBT): Reduces fear of recurrence by 50% in 8-week programs.
- Support Groups: CancerCare’s stomach cancer network offers free virtual sessions.
- Mindfulness Apps: Headspace and Calm reduce anxiety scores by 30% (2022 Psycho-Oncology study).
Q9: Can exercise reduce stomach cancer risk?
A9: Yes. Regular activity:
- Lowers visceral fat (a source of pro-inflammatory cytokines).
- Reduces risk by 20% with 150+ mins/week of moderate exercise (NCI cohort data).
Best Options: Swimming, cycling, or yoga to avoid post-surgical strain.
Q10: What post-treatment complications should survivors monitor?
A10: Common issues include:
- Dumping Syndrome: Managed with protein-focused mini-meals and acarbose medication.
- Osteoporosis: Screen via DEXA scans; supplement calcium/vitamin D.
- Bile Reflux: Treated with ursodeoxycholic acid or Roux-en-Y surgery.
Conclusion
A stomach cancer diagnosis is life-changing, but advancements in treatment and supportive care are improving survival and quality of life. By staying informed, advocating for personalized care, and leaning on community resources, patients can navigate this journey with resilience.
For more guidance, consult your oncologist or visit trusted sources like the National Cancer Institute or CancerCare.

