The thyroid gland, a butterfly-shaped organ at the base of your neck, plays a vital role in regulating metabolism, heart rate, and energy levels. When abnormal cells grow uncontrollably here, thyroid cancer develops. While it’s relatively rare (3% of U.S. cancer cases), early detection and treatment are critical for positive outcomes. Below, we break down its types, warning signs, and the latest therapies.
Types of Thyroid Cancer
Thyroid cancer is classified based on the cell type from which it originates, its behavior, and genetic characteristics. Understanding these distinctions is critical for prognosis and treatment planning. Below, we explore the four primary types in depth:
1. Differentiated Thyroid Cancer (90% of Cases)
These cancers develop from thyroid follicular cells (which produce thyroid hormones) and retain some ability to resemble normal thyroid tissue under a microscope. They are further divided into two subtypes:
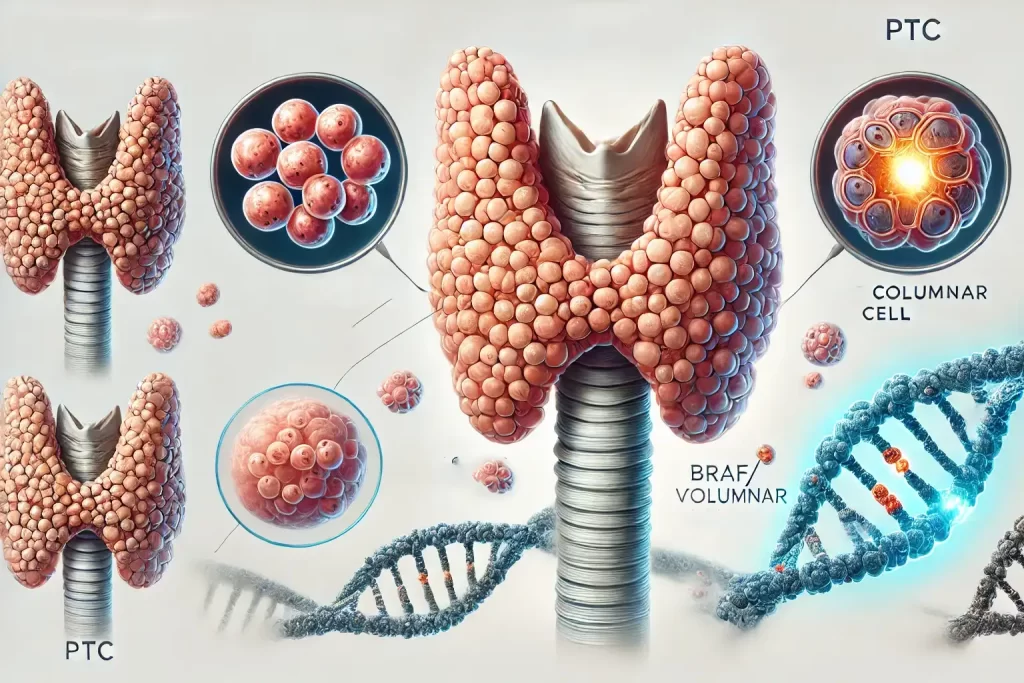
a. Papillary Thyroid Carcinoma (PTC)
- Prevalence: 80–85% of all thyroid cancers.
- Characteristics:
- Slow-growing, often confined to the thyroid.
- Distinctive features: Psammoma bodies (calcified deposits) and nuclear grooves visible under microscopy.
- Common genetic mutations: BRAF V600E (50–80% of cases) and RET/PTC rearrangements.
- Prognosis: Excellent, with a 10-year survival rate exceeding 95% for localized cases.
- Risk Factors: Radiation exposure (e.g., childhood head/neck radiation).
- Subtypes:
- Follicular Variant: Mimics follicular carcinoma but behaves like PTC.
- Tall Cell/Columnar Cell: More aggressive, higher recurrence risk.
b. Follicular Thyroid Carcinoma (FTC)
- Prevalence: 10–15% of thyroid cancers.
- Characteristics:
- Spreads via vascular invasion (entering blood vessels), potentially metastasizing to bones or lungs.
- Subtypes include Hurthle Cell Carcinoma, known for large eosinophilic cells and a higher recurrence rate.
- Genetic drivers: RAS mutations and PAX8/PPARγ rearrangements.
- Diagnosis: Requires identification of capsular or vascular invasion (often challenging on biopsy).
- Prognosis: 10-year survival ~85% for localized cases, dropping to 40% if distant metastases occur.
2. Medullary Thyroid Cancer (MTC) (4% of Cases)
- Origin: Arises from parafollicular C cells (neuroendocrine cells that produce calcitonin).
- Genetic Links:
- Hereditary (25%): Associated with Multiple Endocrine Neoplasia type 2 (MEN2) syndromes (MEN2A/MEN2B) caused by RET proto-oncogene mutations.
- Sporadic (75%): No family history, but 50% have somatic RET mutations.
- Diagnosis: Elevated serum calcitonin and carcinoembryonic antigen (CEA). Genetic testing for RET mutations is standard.
- Symptoms: Chronic diarrhea (due to calcitonin secretion) or flushing in advanced cases.
- Prognosis: 10-year survival ranges from 75% (localized) to 20% (metastatic).
3. Anaplastic Thyroid Cancer (ATC) (1–2% of Cases)
- Nature: Undifferentiated, aggressive, and often fatal.
- Origins:
- May arise de novo or from dedifferentiation of existing papillary/follicular cancers.
- Hallmarked by p53 mutations and loss of thyroid-specific markers (e.g., thyroglobulin).
- Behavior:
- Rapid growth, often invading the trachea, esophagus, or carotid artery.
- Distant metastases (lungs, brain) in 50% of cases at diagnosis.
- Symptoms: Sudden neck mass, stridor, dysphagia, or vocal cord paralysis.
- Prognosis: Median survival is 3–6 months. 1-year survival <20%.
- Emerging Treatments:
- Targeted therapies (e.g., BRAF/MEK inhibitors for tumors with BRAF mutations).
- Immunotherapy (e.g., pembrolizumab) in clinical trials.
4. Rare Thyroid Cancers (<1%)
- Thyroid Lymphoma: Develops from immune cells; linked to Hashimoto’s thyroiditis.
- Squamous Cell Carcinoma: Extremely rare, often misdiagnosed as anaplastic cancer.
Comparison Table: Key Features of Thyroid Cancer Types
| Type | Cell Origin | Common Mutations | 10-Year Survival | Metastasis Sites |
|---|---|---|---|---|
| Papillary Carcinoma | Follicular cells | BRAF, RET/PTC | >95% (localized) | Lymph nodes, lungs |
| Follicular Carcinoma | Follicular cells | RAS, PAX8/PPARγ | ~85% (localized) | Bones, lungs |
| Medullary Cancer | Parafollicular C | RET | 75% (localized) | Liver, lungs |
| Anaplastic Cancer | Dedifferentiated | TP53, BRAF | <20% (all stages) | Lungs, brain, bones |
Emerging Research & Precision Medicine
- Papillary/Follicular: Focus on reducing overtreatment (e.g., active surveillance for microcarcinomas <1 cm).
- Medullary: RET inhibitors (selpercatinib, pralsetinib) show 70% response rates in RET-mutant MTC.
- Anaplastic: Combination therapies (BRAF/MEK inhibitors + immunotherapy) are being trialed to improve survival.
Who’s at Risk? Key Risk Factors
Thyroid cancer’s risk profile is shaped by a mix of demographic, environmental, and genetic factors. While anyone can develop thyroid cancer, certain groups face significantly higher risks. Below, we unpack these factors in detail, including the biological mechanisms and supporting data.
1. Gender Disparity: Women at 3x Higher Risk
- Statistics: Women account for 75% of thyroid cancer cases, with a lifetime risk of 1 in 56 (vs. 1 in 176 for men).
- Why Women?
- Hormonal Influence: Estrogen receptors on thyroid cells may promote tumor growth. Pregnancy and menopause-related hormonal shifts are also linked to nodule formation.
- Diagnostic Bias: Women are more likely to undergo neck imaging (e.g., for thyroid nodules during routine exams), leading to higher detection rates.
- Cancer Type Differences:
- Papillary carcinoma (most common type) is 3x more frequent in women.
- Medullary and anaplastic cancers show less gender disparity.
2. Age: Most Common in Adults 25–65, But Varies by Type
- Peak Incidence:
- Papillary/Follicular: Diagnosed most often in 30–50-year-olds.
- Medullary: Typically appears in 40–60-year-olds (sporadic cases) or 20–30-year-olds (hereditary MEN2-related).
- Anaplastic: Usually occurs in adults over 65.
- Why Younger Adults?
- Radiation exposure in childhood/teen years has a latency period of 10–30 years.
- Genetic mutations (e.g., RET) may manifest earlier in life.
3. Radiation Exposure: The Strongest Environmental Risk
- Sources of Exposure:
- Medical Radiation: Childhood radiation therapy for Hodgkin’s lymphoma, tonsils, or acne (common pre-1960s).
- Environmental: Nuclear fallout (e.g., Chernobyl, Fukushima survivors) or occupational exposure (e.g., radiologists).
- Mechanism:
- Ionizing radiation damages thyroid cell DNA, triggering mutations (e.g., RET/PTC rearrangements in papillary cancer).
- Children’s thyroids are 10x more radiation-sensitive due to rapid cell division.
- Risk Magnitude:
- A single 10–1500 mGy dose increases thyroid cancer risk by 50–500%.
- Post-Chernobyl, papillary thyroid cancer rates surged 100x in exposed children.
4. Family History & Genetic Syndromes
a. Hereditary Syndromes
- Multiple Endocrine Neoplasia Type 2 (MEN2):
- Risk: Nearly 100% lifetime risk of medullary thyroid cancer (MTC).
- Genetics: Caused by RET proto-oncogene mutations. MEN2A also causes pheochromocytoma and hyperparathyroidism.
- Familial Medullary Thyroid Cancer (FMTC):
- A MEN2 variant with isolated MTC risk.
- Other Syndromes:
- Cowden Syndrome (PTEN mutations): 10–35% risk of follicular/papillary thyroid cancer.
- DICER1 Syndrome: Linked to thyroid nodules and rare cancers.
b. Family History of Thyroid Disease
- First-degree relatives of thyroid cancer patients have a 4–6x higher risk.
- Non-cancerous thyroid conditions (e.g., goiter, Hashimoto’s thyroiditis) also correlate with increased cancer risk.
5. Additional Risk Factors
a. Iodine Intake
- Low Iodine Diets: Linked to follicular thyroid cancer.
- Excess Iodine: May increase papillary cancer risk in genetically predisposed individuals.
b. Obesity
- Obesity (BMI ≥30) raises thyroid cancer risk by 30%, possibly due to insulin resistance and chronic inflammation.
c. Ethnicity & Geography
- Higher Incidence in:
- Asian populations (potentially due to iodine-rich diets and genetic factors).
- Developed countries (likely due to increased diagnostic scrutiny).
Risk Factor Comparison Table
| Factor | Associated Risk Increase | Linked Cancer Types | Key Mechanism |
|---|---|---|---|
| Female Gender | 3x higher vs. men | Papillary, Follicular | Hormonal (estrogen signaling) |
| Childhood Radiation | 5–15x higher | Papillary | DNA damage → RET/PTC mutations |
| MEN2 Syndrome | Near 100% risk | Medullary | RET germline mutations |
| Family History | 4–6x higher | All types | Shared genetics/environment |
| Obesity (BMI ≥30) | 1.3x higher | Papillary | Insulin/IGF-1 pathway activation |
Prevention Strategies for High-Risk Groups
- Genetic Testing: Recommended for families with MEN2 or FMTC. Prophylactic thyroidectomy may be advised for RET mutation carriers.
- Radiation Mitigation: Avoid unnecessary CT scans in children; use thyroid shields during X-rays.
- Screening: Annual neck ultrasounds for those with family history or prior radiation exposure.
Emerging Research on Risk Factors
- Gene-Environment Interactions: How BRAF mutations synergize with radiation in papillary cancer.
- Epigenetics: DNA methylation changes in obesity-related thyroid tumors.
Symptoms: When to See a Doctor
Thyroid cancer often begins silently, with many cases detected incidentally during routine exams. However, as the disease progresses, specific symptoms may emerge. Understanding these signs and their implications is critical for timely diagnosis and treatment. Below is a detailed exploration of thyroid cancer symptoms, their underlying causes, and actionable guidance for seeking medical care.
1. Neck Lump or Swelling
- Description: A palpable nodule or mass in the anterior neck, typically painless and firm.
- Key Details:
- Prevalence: Thyroid nodules are found in ~50% of adults over 50 via ultrasound, but only 5–15% are cancerous.
- Suspicious Features: Rapid growth, irregular borders, fixation to surrounding tissues, or associated lymph node enlargement.
- Benign vs. Malignant: Benign nodules (e.g., cysts, adenomas) often feel soft and mobile, while cancerous ones may be hard and static.
Why It Happens:
Cancerous cells form a discrete mass within the thyroid gland. Larger tumors (>1 cm) are more likely to be palpable.
2. Hoarseness or Voice Changes (Lasting >2 Weeks)
- Description: Persistent raspiness, weakness, or loss of voice unresponsive to rest or antibiotics.
- Mechanism:
- The recurrent laryngeal nerve, which controls vocal cords, runs behind the thyroid. Tumors (especially anaplastic or advanced papillary cancers) may compress or invade this nerve.
- Direct tumor extension into the larynx or trachea can also alter voice quality.
Red Flags:
- Voice changes lasting >2 weeks with no history of infection or overuse.
- Progressive worsening despite conservative measures.
3. Difficulty Swallowing (Dysphagia) or Breathing (Dyspnea)
- Description:
- Dysphagia: Feeling of food “sticking” in the throat.
- Dyspnea: Shortness of breath, especially when lying flat.
- Causes:
- Mechanical compression of the esophagus or trachea by a large tumor.
- Invasion into surrounding structures (e.g., trachea, mediastinum).
Emergency Signs:
- Sudden-onset stridor (high-pitched breathing sound) or choking, indicating acute airway obstruction.
4. Persistent Neck/Throat Pain
- Description: Dull ache or sharp pain localized to the thyroid region, sometimes radiating to the jaw or ears.
- Associated Conditions:
- Rare in early-stage cancer but may occur with aggressive subtypes (e.g., anaplastic thyroid cancer) or inflammation from rapid tumor growth.
- Differentiate from thyroiditis (e.g., Hashimoto’s), which causes diffuse tenderness.
When to See a Doctor: Red Flags
Consult a healthcare provider if you experience:
- A neck lump that grows rapidly or is accompanied by swollen lymph nodes.
- Voice changes persisting beyond 2 weeks.
- Progressive difficulty swallowing or breathing.
- Unexplained weight loss, fatigue, or night sweats (systemic symptoms).
Diagnostic Evaluation: What to Expect
- Physical Exam: Palpation of the neck for nodules, lymphadenopathy, or tracheal deviation.
- Imaging:
- Ultrasound: Assesses nodule size, shape, and vascularity. Hypoechoic nodules with microcalcifications or irregular margins are suspicious.
- CT/MRI: Evaluates tumor extension into the chest or airway.
- Biopsy:
- Fine-Needle Aspiration (FNA): Extracts cells from the nodule for cytology.
- Molecular Testing: BRAF, RET, or RAS mutations may confirm malignancy.
- Blood Tests:
- TSH, thyroglobulin (for differentiated cancers).
- Calcitonin (elevated in medullary thyroid cancer).
Benign vs. Malignant Nodules: Key Statistics
| Feature | Benign Nodule | Malignant Nodule |
|---|---|---|
| Prevalence | 85–95% of all nodules | 5–15% |
| Growth Rate | Slow or stable | Rapid |
| Ultrasound Markers | Spongiform, cystic | Hypoechoic, microcalcifications |
| Pain | Rare (unless inflamed) | Rare (except in anaplastic) |
Psychological Considerations
While most nodules are benign, persistent symptoms warrant evaluation to rule out malignancy. Avoid self-diagnosis; imaging and biopsy provide clarity. For high-risk groups (e.g., radiation exposure, RET carriers), regular screening is crucial.

Conclusion
Thyroid cancer symptoms are often nonspecific but become concerning when persistent or progressive. Early consultation with an endocrinologist or ENT specialist ensures timely diagnosis. Remember: Most nodules are harmless, but vigilance saves lives.
Take Action:
- Schedule a neck ultrasound if you notice a lump or voice changes.
- Discuss family history or radiation exposure with your doctor.
By understanding these symptoms and their implications, you empower yourself to seek care at the earliest, most treatable stage.
How Thyroid Cancer is Diagnosed
Thyroid cancer diagnosis is a meticulous, multi-step process that integrates clinical evaluation, advanced imaging, biopsy techniques, and laboratory tests. Here’s a detailed breakdown of each stage:
1. Physical Examination: The First Clue
- Technique:
- Palpation: The clinician systematically feels the neck for thyroid nodules, assessing their size, texture (soft vs. firm), mobility (fixed vs. movable), and location.
- Lymph Node Check: Examines cervical lymph nodes (e.g., lateral neck chains) for enlargement, which may indicate metastasis.
- Concerning Findings:
- Hard, irregular nodules fixed to surrounding tissues.
- Rapidly growing masses or lymphadenopathy.
- Limitations: Small nodules (<1 cm) or posteriorly located tumors may evade detection by palpation alone.
2. Imaging: Visualizing the Thyroid and Beyond
a. Thyroid Ultrasound
- Purpose: First-line imaging to evaluate nodule characteristics and guide biopsy.
- Key Features Analyzed:
- Echogenicity: Hypoechoic nodules (darker than surrounding tissue) are suspicious.
- Margins: Irregular or spiculated borders suggest malignancy.
- Microcalcifications: Punctate bright spots linked to papillary carcinoma.
- Vascularity: Hypervascularity may indicate aggressive tumors.
- TI-RADS Scoring: Categorizes nodules from TR1 (benign) to TR5 (highly suspicious) based on ultrasound features.
b. Cross-Sectional Imaging (CT/MRI)
- Indications:
- Assess extrathyroidal extension (e.g., tracheal, esophageal invasion).
- Evaluate retrosternal goiters or metastatic disease (lungs, mediastinum).
- CT with Contrast: Highlights vascular invasion but delays radioactive iodine therapy due to iodine load.
- MRI: Superior for soft tissue resolution; used if anaplastic cancer or spinal involvement is suspected.
c. Radioactive Iodine Scan (RAI)
- Use Case: Primarily for post-diagnosis staging in differentiated thyroid cancer (papillary/follicular).
- Mechanism: Thyroid cells absorb iodine; “cold” nodules (non-uptake) are more likely malignant.
3. Biopsy: Fine-Needle Aspiration (FNA)
- Procedure:
- Performed under ultrasound guidance for precision.
- A thin needle extracts cells from the nodule for cytopathological analysis.
- Bethesda System for Cytopathology:
- Categories:
- I (Non-diagnostic): Repeat FNA needed.
- II (Benign): 0–3% cancer risk.
- III–IV (Atypia/Follicular Neoplasm): 5–30% risk.
- V–VI (Suspicious/Malignant): 50–100% risk.
- Categories:
- Molecular Testing:
- BRAF V600E, RET/PTC, RAS: Confirm malignancy in indeterminate nodules (Bethesda III–IV).
- Calcitonin Immunostaining: Diagnoses medullary thyroid cancer.
4. Blood Tests: Biomarkers and Hormonal Insights
- Calcitonin:
- Role: Gold standard for medullary thyroid cancer (MTC). Levels >100 pg/mL strongly indicate MTC.
- Provocative Testing: Pentagastrin stimulation for borderline cases.
- Thyroglobulin (Tg):
- Use: Monitors recurrence in differentiated thyroid cancer post-thyroidectomy.
- Diagnostic Caveat: Not used preoperatively, as benign nodules also produce Tg.
- Carcinoembryonic Antigen (CEA): Elevated in advanced MTC.
- TSH: Assess thyroid function; suppressed TSH may indicate hyperfunctioning nodules (rarely cancerous).
5. Advanced Diagnostic Tools
- Laryngoscopy: Evaluates vocal cord paralysis caused by recurrent laryngeal nerve invasion.
- Genetic Testing:
- RET Proto-Oncogene: Identifies hereditary MTC in MEN2 syndrome.
- DICER1/PTEN: For familial non-medullary thyroid cancer syndromes.
6. Staging and Metastatic Workup
- TNM Staging: Integrates tumor size (T), lymph node involvement (N), and distant metastases (M).
- Imaging for Metastases:
- Chest CT: Detects lung metastases.
- FDG-PET/CT: For aggressive subtypes (e.g., anaplastic) or radioiodine-refractory disease.
Challenges and Follow-Up
- Indeterminate Biopsies: Molecular testing (e.g., ThyroSeq, Afirma) refines risk stratification.
- False Negatives: Repeat FNA or lobectomy for high-suspicion nodules.
- Patient Risk Factors: Radiation history or genetic syndromes warrant lifelong surveillance.
Conclusion
Thyroid cancer diagnosis hinges on a synergy of clinical suspicion, imaging precision, cytopathological expertise, and biomarker analysis. Early and accurate diagnosis, guided by protocols like TI-RADS and Bethesda, ensures timely intervention, optimizing outcomes for this highly treatable malignancy.
Modern Treatment Options
Treatment dependThyroid cancer treatment has evolved significantly, emphasizing personalized approaches based on tumor type, stage, genetic profile, and patient preferences. Below, we dissect the latest strategies, from surgery to cutting-edge targeted therapies, with a focus on efficacy, risks, and emerging trends.
1. Surgery: The Foundation of Curative Treatment
a. Thyroidectomy (Partial vs. Total)
- Lobectomy:
- Indications: Small (<1 cm), low-risk papillary microcarcinomas confined to one lobe.
- Advantages: Preserves thyroid function in 20–30% of patients, avoiding lifelong hormone replacement.
- Limitations: 5–10% risk of recurrence in residual lobe.
- Total Thyroidectomy:
- Indications: Tumors >4 cm, bilateral nodules, aggressive subtypes (e.g., anaplastic), or familial cases (MEN2).
- Complications: Hypocalcemia (10–15% temporary, 1–3% permanent), recurrent laryngeal nerve injury (1–2%).
- Nerve Monitoring: Intraoperative neuromonitoring reduces vocal cord paralysis risk.
b. Lymph Node Dissection
- Central Neck Dissection (Level VI):
- Performed for clinically involved nodes or high-risk tumors (e.g., T3/T4).
- Reduces recurrence but increases hypoparathyroidism risk.
- Lateral Neck Dissection (Level II–V):
- Reserved for palpable or imaging-confirmed lateral lymph node metastases.
Robotic/Transoral Approaches: Minimally invasive techniques reduce scarring, ideal for young patients.
2. Radioactive Iodine (RAI) Therapy
a. Mechanism
- Differentiated thyroid cancers (papillary/follicular) absorb iodine-131, which emits beta radiation to destroy residual cancer cells.
b. Dosing Strategies
- Low-Dose (30–100 mCi): For intermediate-risk patients with microscopic residual disease.
- High-Dose (100–200 mCi): For distant metastases or gross residual tumor.
c. Emerging Debates
- De-Escalation: Recent ATA guidelines recommend against RAI for low-risk tumors (e.g., <1 cm, unifocal), reducing long-term leukemia/secondary cancer risks.
- Preparation: Thyroid hormone withdrawal vs. recombinant TSH (Thyrogen®) to elevate TSH levels.
3. Targeted Drug Therapies
a. Kinase Inhibitors
- Lenvatinib (Lenvima®):
- Targets VEGF receptors 1–3, FGFR 1–4, RET, and KIT.
- Efficacy: 65% response rate in RAI-refractory differentiated thyroid cancer.
- Side Effects: Hypertension (70%), fatigue, hand-foot syndrome.
- Sorafenib (Nexavar®):
- Inhibits BRAF, VEGFR, and PDGFR.
- PFS benefit of 10.8 vs. 5.8 months (placebo).
b. RET Inhibitors
- Selpercatinib (Retevmo®) and Pralsetinib (Gavreto®):
- For RET-mutant medullary thyroid cancer (MTC).
- Response Rates: 70–80% in advanced MTC.
c. BRAF/MEK Inhibitors
- Dabrafenib + Trametinib:
- For BRAF V600E-mutant anaplastic thyroid cancer (ATC).
- Doubles 1-year survival (50% vs. 25% historical).
4. Watchful Waiting (Active Surveillance)
- Criteria: Papillary microcarcinomas (<1 cm), no extrathyroidal extension, or high surgical risk patients.
- Protocol:
- Ultrasound every 6–12 months to monitor nodule growth (>3 mm/year triggers intervention).
- Japan’s Kuma Hospital data: 10-year progression-free survival >95% with surveillance.
5. Thyroid Hormone Replacement
- Levothyroxine:
- TSH Suppression: For high-risk patients (TSH <0.1 mU/L) to reduce recurrence.
- Replacement Dose: For low-risk cases (TSH 0.5–2.0 mU/L).
- Monitoring: Annual TSH/fT4 tests and bone density scans (long-term suppression risks osteoporosis).
6. Emerging & Experimental Therapies
- Immunotherapy:
- Pembrolizumab (anti-PD1) trials for anaplastic thyroid cancer show 20% response rates.
- Peptide Receptor Radionuclide Therapy (PRRT): For somatostatin receptor-positive MTC.
- Redifferentiation Agents: Drugs like selumetinib restore iodine uptake in RAI-refractory tumors.
Treatment Selection by Thyroid Cancer Type
| Cancer Type | First-Line Treatment | Advanced/Recurrent Options |
|---|---|---|
| Papillary | Lobectomy/RAI | Lenvatinib, RAI re-treatment |
| Follicular | Total thyroidectomy + RAI | Sorafenib, clinical trials |
| Medullary (MTC) | Total thyroidectomy + LN dissection | Selpercatinib, Vandetanib |
| Anaplastic (ATC) | Debulking surgery + chemoradiation | Dabrafenib/Trametinib, immunotherapy |
Key Considerations for Patients
- Quality of Life: Balance curative intent with risks (e.g., voice changes, hypocalcemia).
- Genetic Testing: Essential for hereditary MTC (MEN2) to guide prophylactic surgery.
- Clinical Trials: Encourage participation for refractory cases (e.g., NCT03181100 for ATC).
Conclusion
Modern thyroid cancer treatment prioritizes precision, leveraging surgery, RAI, and molecular therapies tailored to tumor biology. With 10-year survival exceeding 95% for localized disease, early diagnosis and multidisciplinary care remain paramount. Patients should seek centers with expertise in thyroid oncology to navigate evolving options like immunotherapy and redifferentiation strategies.
Living with Thyroid Cancer: Recovery & Support
Post-treatment life after thyroid cancer requires meticulous monitoring, adaptive lifestyle changes, and emotional resilience. Below, we delve into structured recovery protocols, psychological support systems, and cutting-edge research shaping survivorship.
1. Post-Treatment Surveillance: Preventing Recurrence
a. Hormone Monitoring & TSH Suppression
- Levothyroxine Management:
- High-Risk Patients: Maintain TSH <0.1 mU/L to inhibit residual cancer growth.
- Low-Risk Patients: Target TSH 0.5–2.0 mU/L to balance recurrence risk and bone/cardiac health.
- Blood Tests:
- Every 3–6 Months: TSH, free T4, thyroglobulin (Tg) for differentiated cancers.
- Calcitonin/CEA: Every 6 months for medullary thyroid cancer (MTC) survivors.
b. Imaging Surveillance
- Neck Ultrasound:
- Frequency: Every 6–12 months for 5 years, then annually. High-risk cases require 6-month intervals.
- Focus: Thyroid bed, cervical lymph nodes (Levels II–VI).
- Advanced Imaging:
- FDG-PET/CT: For Tg-positive, RAI-negative patients to locate metastases.
- DxWBS (Diagnostic Whole-Body Scan): Post-RAI therapy to assess iodine avidity.
c. Emerging Monitoring Tools
- Liquid Biopsies: Detect circulating tumor DNA (e.g., BRAF, RET mutations) for early recurrence signs.
- Thyroglobulin Antibodies (TgAb): Rising titers may indicate recurrence in antibody-positive patients.
2. Survivorship Challenges & Coping Strategies
a. Physical Health
- Hypothyroidism Symptoms: Fatigue, weight gain, depression—managed via levothyroxine dose adjustments.
- Hypoparathyroidism: Calcium/vitamin D supplementation for post-surgical patients.
- Voice/Vascular Issues:
- Laryngoscopy: For persistent hoarseness (vocal cord paralysis).
- Neck Stiffness: Physical therapy to improve mobility post-dissection.
b. Emotional & Psychological Support
- Support Networks:
- ThyCa: Thyroid Cancer Survivors’ Association: Offers peer-led support groups and educational webinars.
- CancerCare: Free counseling and financial aid for treatment-related costs.
- Mental Health Tools:
- Cognitive Behavioral Therapy (CBT) for anxiety/depression.
- Mindfulness apps (e.g., Headspace) to manage scan-related stress.
3. Prevention Strategies for High-Risk Populations
a. Radiation Risk Mitigation
- Medical Imaging: Use thyroid shields during dental/chest X-rays; opt for MRI over CT in children.
- Environmental Awareness: Potassium iodide (KI) pills for those near nuclear facilities.
b. Genetic Counseling & Prophylactic Surgery
- Who Needs Testing?:
- Families with MEN2, RET mutations, or ≥2 relatives with thyroid cancer.
- RET Carriers: Prophylactic thyroidectomy by age 5 (MEN2B) or 5–10 (MEN2A).
- Preimplantation Genetic Diagnosis (PGD): For families with hereditary MTC to prevent transmission.
4. Breakthrough Research & Clinical Trials
a. Immunotherapy Advancements
- Anaplastic Thyroid Cancer (ATC):
- Pembrolizumab + Lenvatinib: Phase II trials show 33% response rate and 9.7-month median survival.
- CAR T-Cell Therapy: Early-stage trials targeting thyroglobulin or calcitonin.
- Differentiated Cancers:
- Combination RAI + MEK Inhibitors: Redifferentiation strategies to restore iodine uptake.
b. Precision Genetic Testing
- Next-Generation Sequencing (NGS) Panels:
- ThyroSeq v3: 112-gene panel for indeterminate nodules (94% accuracy).
- Guardant Reveal: Liquid biopsy detecting RET, BRAF, and RAS mutations.
- FDA Approvals (2023):
- Selpercatinib: Expanded for RET-fusion-positive thyroid cancers.
5. Lifestyle Modifications for Recovery
- Diet:
- Iodine Balance: Avoid excess seaweed/iodized salt for RAI candidates.
- Calcium-Rich Foods: Dairy, leafy greens for post-thyroidectomy patients.
- Exercise: Yoga/Pilates to improve neck mobility and reduce fatigue.
Key Resources:
- American Thyroid Association: Survivorship Guidelines
- NIH Clinical Trials Database (Search: Thyroid Cancer Immunotherapy)
Conclusion
Living with thyroid cancer demands a proactive, informed approach to surveillance, emotional well-being, and lifestyle adaptation. With advancements in genetic testing and immunotherapy, survivors today have unprecedented tools to manage recurrence risks and thrive. Regular engagement with endocrinologists and support networks ensures holistic care tailored to evolving needs.